New ZILRETTA
NDC Number
65250-0003-01
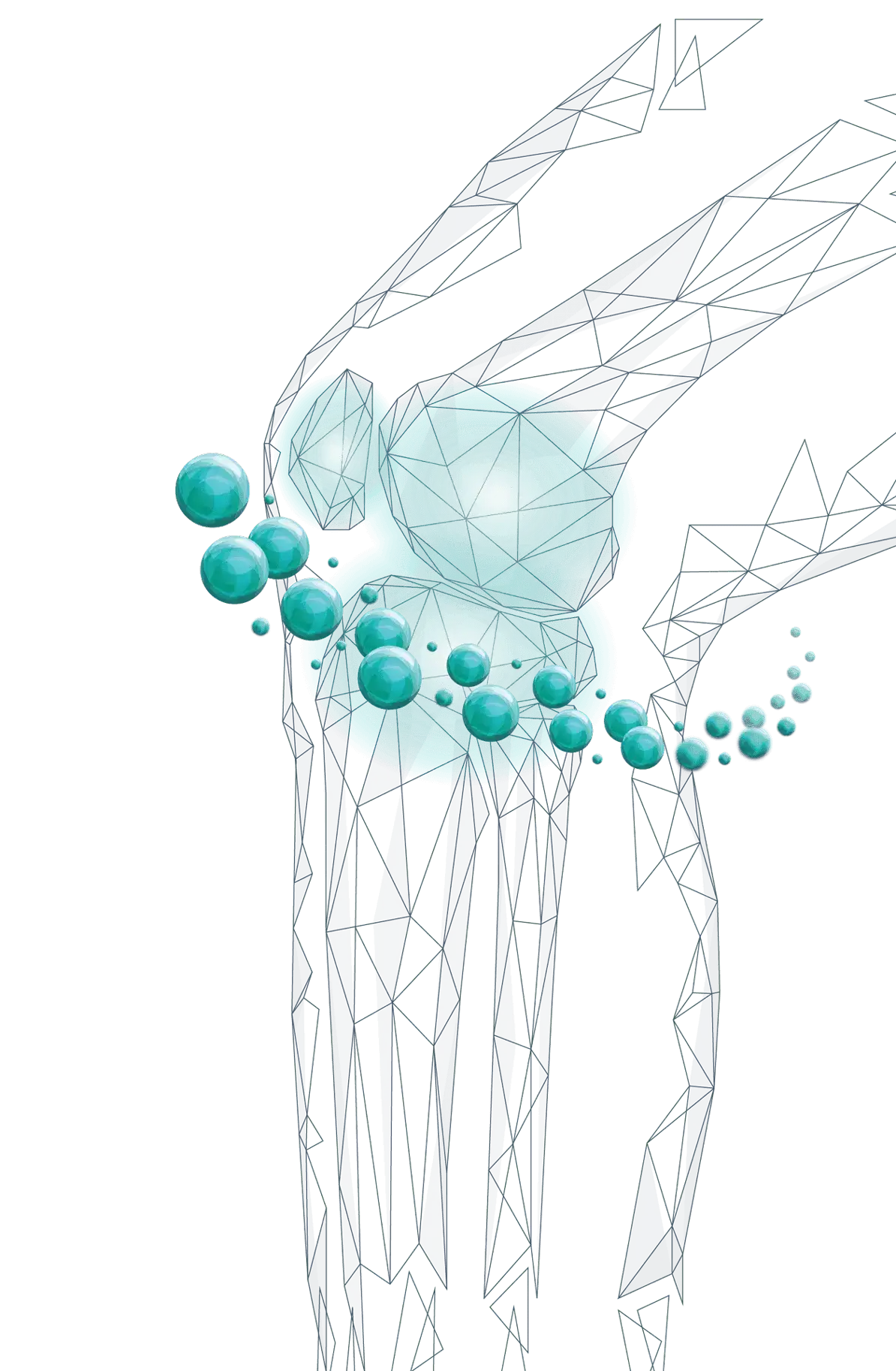
WHY ZILRETTA?
Explore ZILRETTA and learn why it may be right for your patients experiencing OA knee pain.

LOCAL
- Microspheres localize in the synovial tissue, a source of OA knee pain and inflammation1
- In a pharmacokinetic study, TA concentration in the synovial fluid was expressed
through 12
weeks2
- The relevance of the synovial fluid data to the efficacy and safety of ZILRETTA has not been established
RAPID
- 4 days median time to onset with ZILRETTA3*

PERSISTENT
- ≥50% reduction from baseline pain intensity at Week 124
- Significant reduction in pain from Weeks 1-12, extending to Week 163,5

SAFETY
- The overall incidence and nature of adverse reactions were similar to saline control
- Most commonly reported treatment-emergent adverse reactions (incidence ≥1%) in clinical studies were sinusitis, cough, and contusions
*Defined as time from IA injection to the first daily pain assessment of >30% improvement from baseline.
ZILRETTA CAN IMPROVE PATIENT OUTCOMES OVER IMMEDIATE-RELEASE CORTICOSTEROIDS6
Evidence-based review highlights the potential of ZILRETTA to improve outcomes in patients with OA knee pain6
![]() When
we differentiated
intra-articular corticosteroids extended versus immediate release... (Bodick 2015,
Conaghan 2018, and
Langworthy 2019), our analyses demonstrated that, extended release IA steroids can
be used over
immediate release to improve patient outcomes.
When
we differentiated
intra-articular corticosteroids extended versus immediate release... (Bodick 2015,
Conaghan 2018, and
Langworthy 2019), our analyses demonstrated that, extended release IA steroids can
be used over
immediate release to improve patient outcomes.![]() 6†
6†
American Academy of Orthopaedic Surgeons Management of Osteoarthritis of the Knee (Non-Arthroplasty) Evidence-Based Clinical Practice Guideline
†Moderate strength recommendation. Evidence from two or more “Moderate” quality studies with consistent findings, or evidence from a single “High” quality study for recommending for or against the intervention. Also requires no or only minor concerns addressed in the EtD framework.6
Evidence Based
- Based on a systematic review of 25 high and moderate quality studies, the American Academy of Orthopaedic Surgeons (AAOS) updated the evidence-based clinical practice guideline on management of osteoarthritis of the knee (OAK) in August 2021—for the first time since 20136
Differentiated Benefit
- Three key publications (Bodick 2015, Conaghan 2018, and Langworthy 2019) supported the differential analysis indicating benefit of extended-release intra-articular steroids (IAS) over immediate-release corticosteroids to improve patient outcomes6
WANT TO KNOW MORE ABOUT ZILRETTA?
Get more information on ZILRETTA in your office or your inbox. Schedule a rep visit or sign up for email updates to be sure you have the latest information on new developments and support for you, your staff, and your patients.
Request a RepLEARN ABOUT THE EVOLVING ROLE OF ZILRETTA
John Richmond, MD, reviews the clinical data for ZILRETTA and moderates a dynamic panel with Ira Evans, MD, Scott Sigman, MD, and Jason Rand, MD.
Watch The Video
GET YOUR PATIENTS STARTED
We work within your established process and provide comprehensive access support at every step with FlexForward®
SEE HOW IT WORKSIndication
indication and important safety information
References:
- Bodick N, Lufkin J, Willwerth C, et al. An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: a randomized clinical trial. J Bone Joint Surg Am. 2015;97(11):877-888.
- Kraus VB, Conaghan PG, Aazami HA, et al. Synovial and systemic pharmacokinetics (PK) of triamcinolone acetonide (TA) following intra-articular (IA) injection of an extended-release microsphere-based formulation (FX006) or standard crystalline suspension in patients with knee osteoarthritis (OA). Osteoarthr Cartilage. 2018;26(1):34-42.
- Conaghan PG, Hunter DJ, Cohen SB, et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Joint Surg Am. 2018;100(8)666-677.
- Ross E, Katz NP, Conaghan PG, et al. Improved treatment effect of triamcinolone acetonide extended release in patients with concordant baseline pain scores on the average daily pain and Western Ontario and McMaster Universities osteoarthritis index pain scales. Pain Ther. 2022;11(1):289-302.
- Data on file. Pacira Therapeutics, Inc.
- American Academy of Orthopaedic Surgeons Management of Osteoarthritis of the Knee (NonArthroplasty) Evidence-Based Clinical Practice Guideline. https://www.aaos.org/oak3cpg Published August 30, 2021. Accessed March 15, 2023
X

