Trial summary1
- ZILRETTA® (triamcinolone acetonide extended-release injectable
suspension) was studied in a multicenter, international, randomized, double-blind,
parallel-arm, placebo- (saline) and active-controlled (TAcs) trial that evaluated
patients with moderate to severe OA pain of the knee (N=484)
- Patients received a single intra-articular injection of ZILRETTA (32 mg, n=161), saline (n=162), or TAcs (40 mg, n=161) and were followed for up to 24 weeks, with daily pain reported and evaluations at baseline and at Weeks 4, 8, 12, 16, 20, and 24

Pivotal trial outcome measures:
Average daily pain (ADP): Measures daily pain intensity on a 0 to 10 numeric rating scale (NRS)
ADP=average daily pain; OA=osteoarthritis; TAcs=triamcinolone acetonide crystalline suspension.
Phase 3 study
Rapid, persistent, and proven relief from OA knee pain
Patients experienced a significant reduction in OA knee pain vs saline control after a single injection1
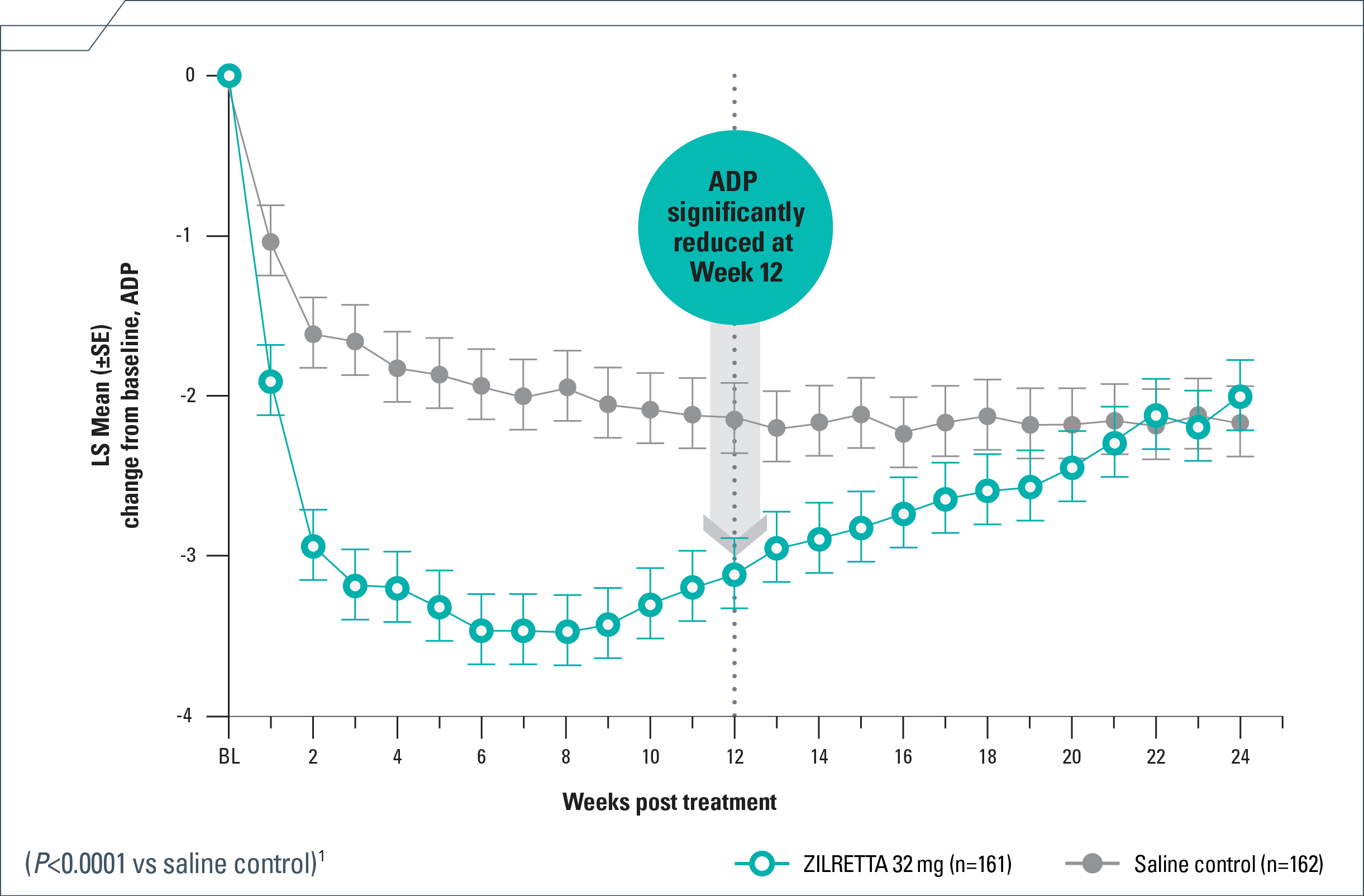
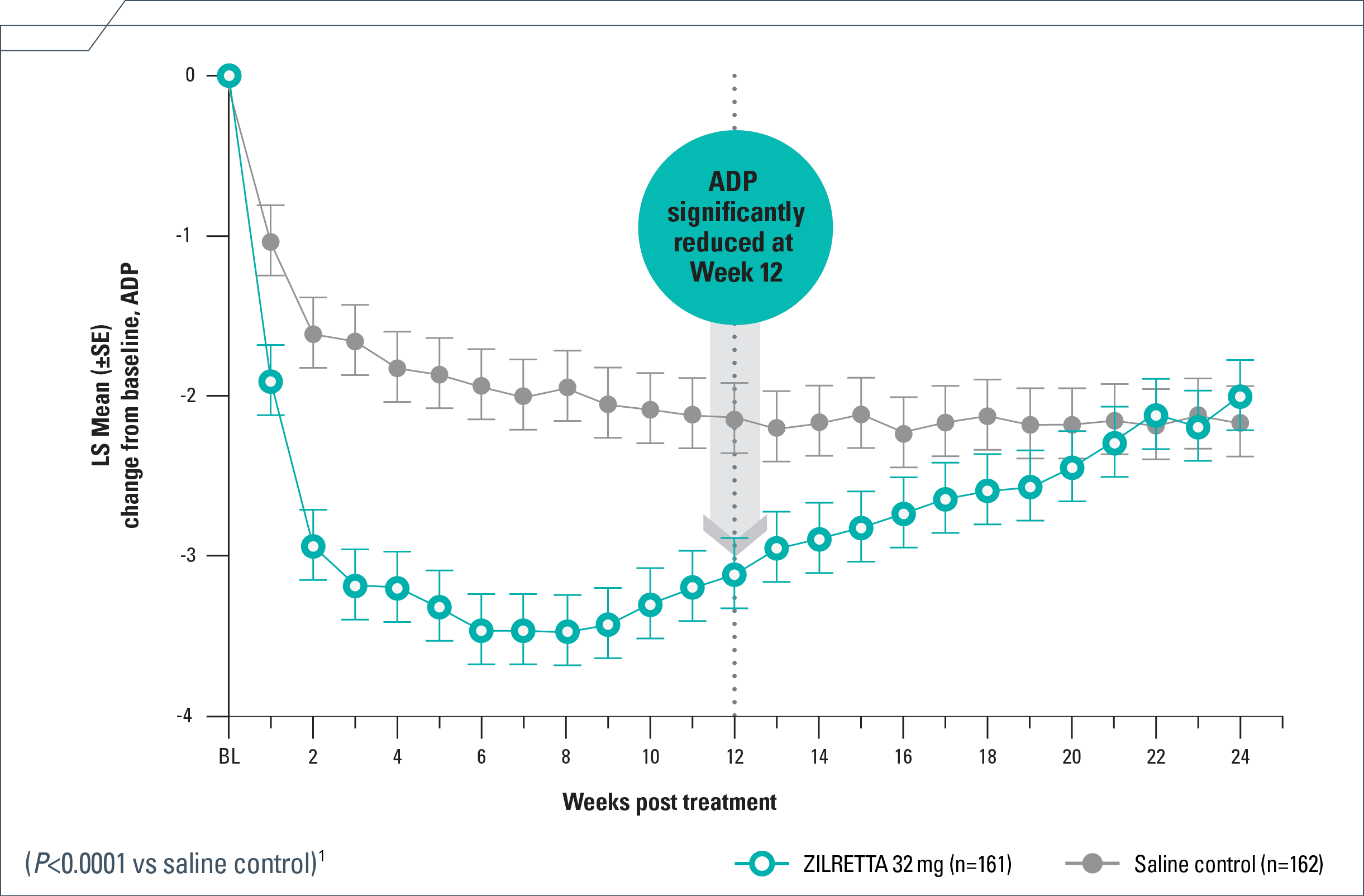
Pain relief in patients with moderate to severe knee OA
- Rapid—4 days median time to onset with ZILRETTA® (triamcinolone acetonide extended-release injectable suspension) and 3 days with TAcs1*
- Substantial—50% reduction from baseline at Week 121
- Persistent—reduction in ADP each week from Weeks 1 to 12, extending to Week 161,4
ADP=average daily pain; BL=baseline; LS=least squares; NRS=numeric rating scale; OA=osteoarthritis; SE=standard error; TAcs=triamcinolone acetonide crystalline suspension.
The majority experienced no/mild knee pain as early as Week 1 and sustained through Week 124
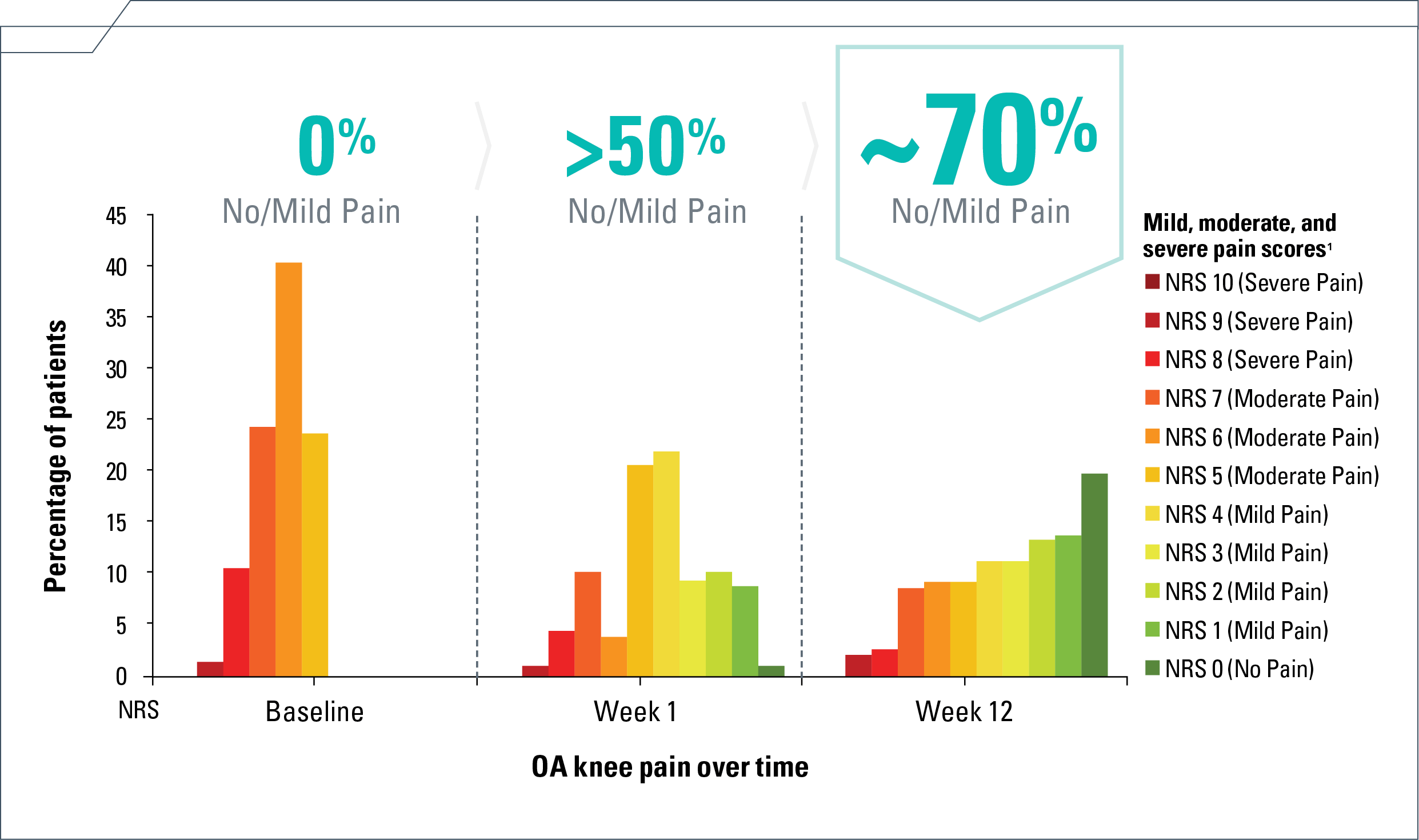
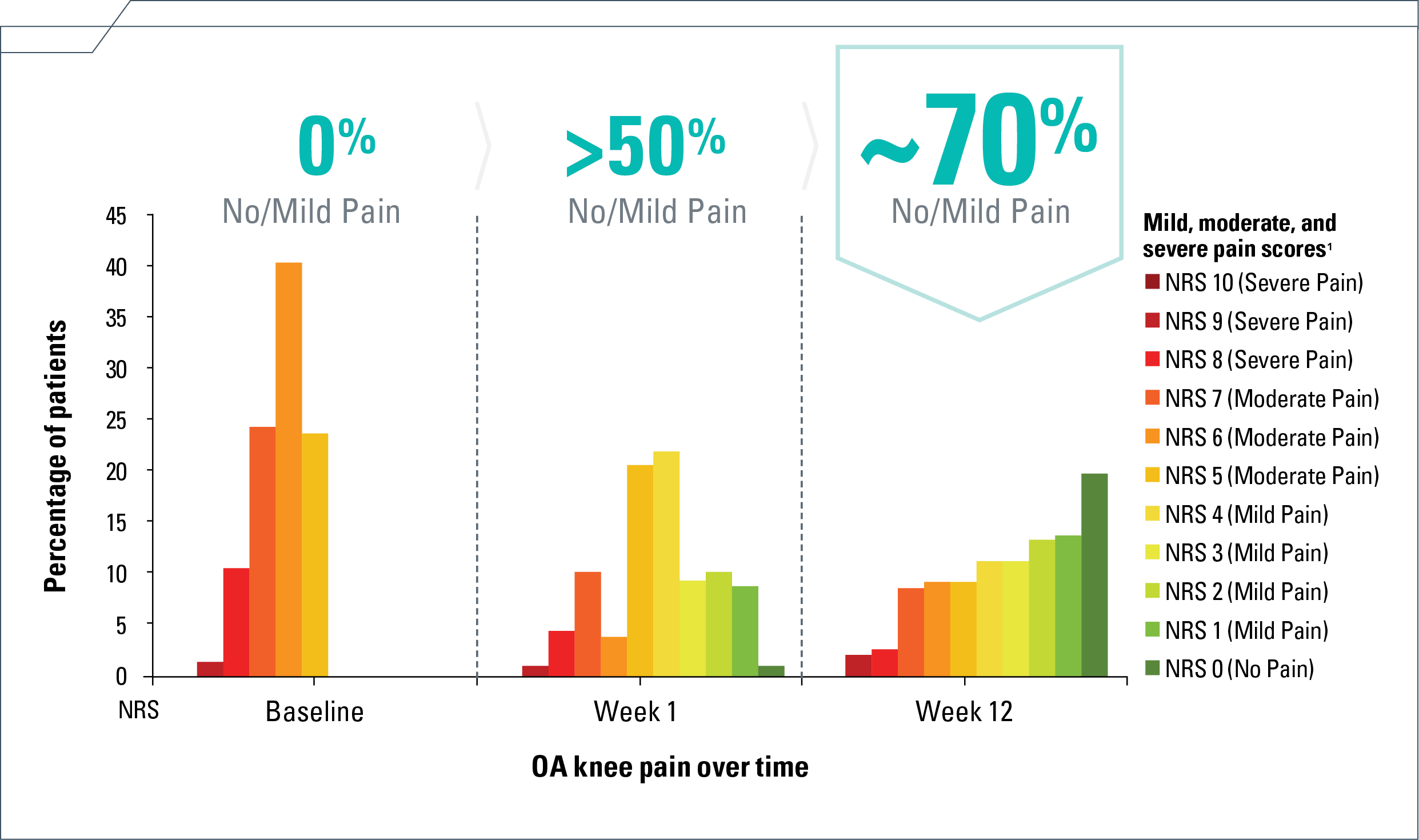
- Percentage of 161 ZILRETTA-treated patients experiencing no/mild knee pain at
Week 4 (21%/49%), Week 8 (22%/47%), and Week 12 (20%/49%), respectively4
NRS=numeric rating scale; OA=osteoarthritis.
ZILRETTA demonstrated clinically meaningful improvement in ADP and WOMAC A pain scores in patients with unilateral OA vs TAcs2
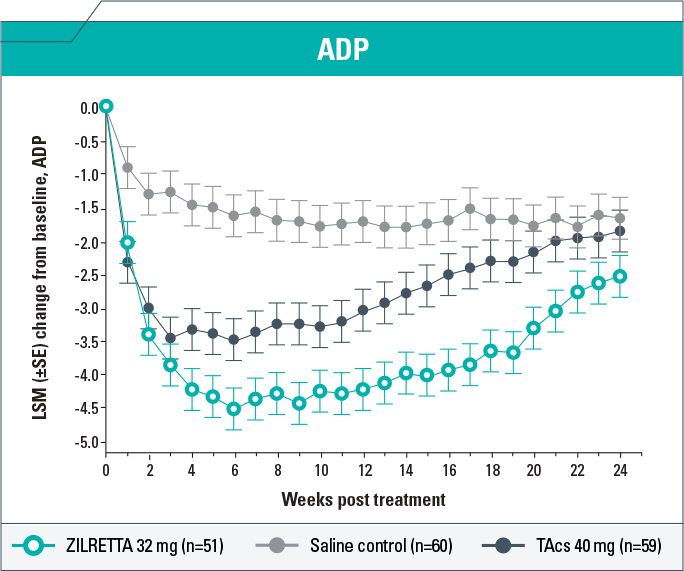
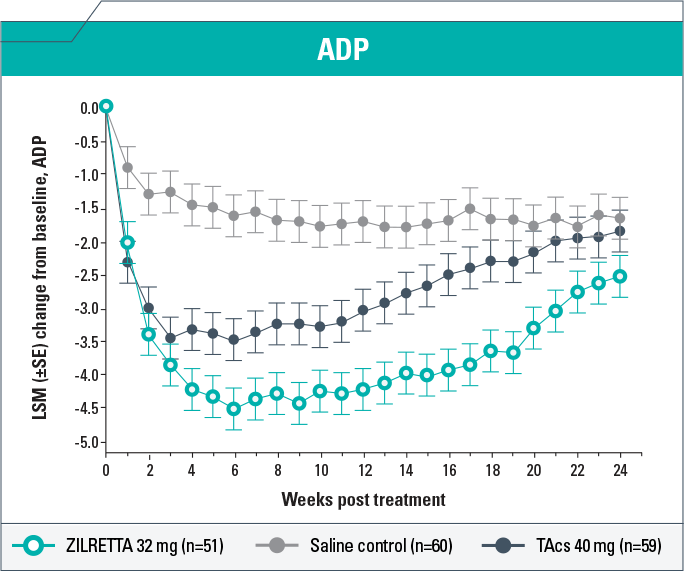
-
ZILRETTA demonstrated clinically meaningful (P<0.05) improvement in ADP intensity compared with saline control from Weeks 1 to 24 and with TAcs from Weeks 4 to 21
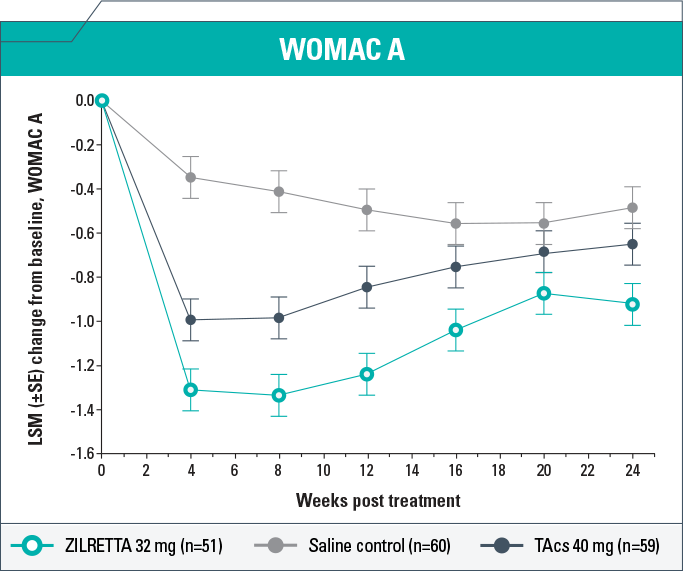

- ZILRETTA demonstrated clinically meaningful (P<0.05) improvements in WOMAC A scores compared with saline control on Weeks 4, 8, 12, and 24 and compared with TAcs on Weeks 4, 8, 12, and 24
Results from a post hoc analysis of a ZILRETTA Phase 3 trial
ADP=average daily pain; LSM=least squares mean; OA=osteoarthritis; SE=standard error; TAcs=triamcinolone acetonide crystalline suspension; WOMAC=Western Ontario and McMaster Universities Osteoarthritis Index.
ZILRETTA demonstrated clinically meaningful improvements in outcomes among patients who reported consistent moderate to severe OA pain in both ADP and WOMAC A scales3
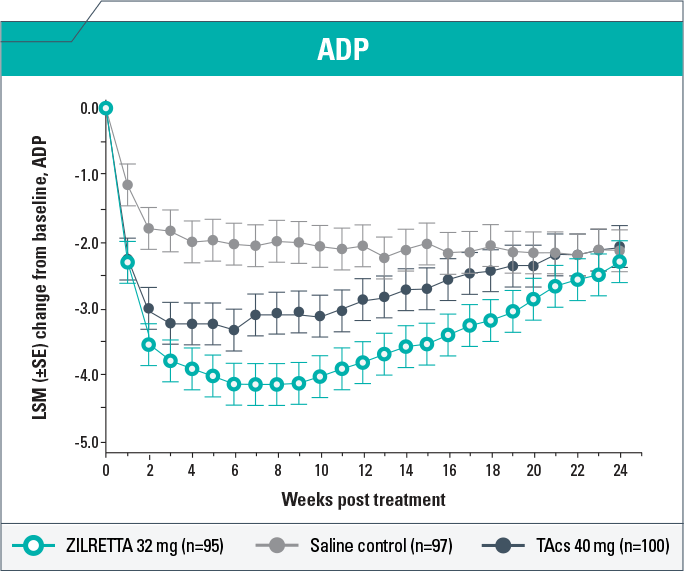
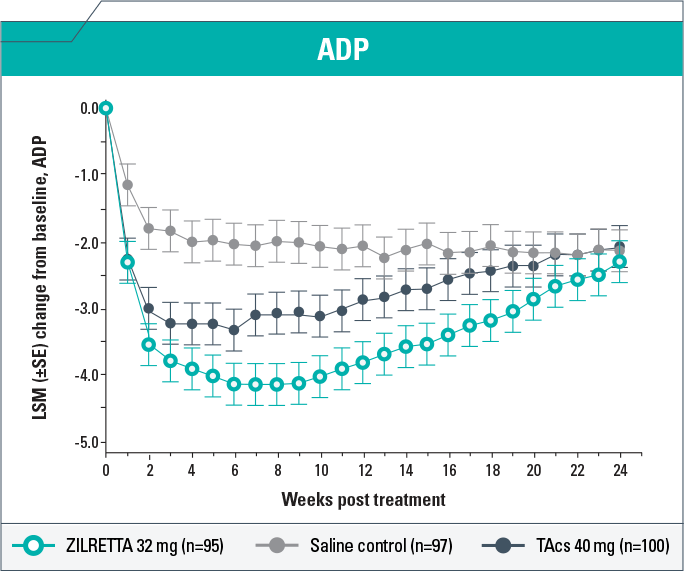
-
Change from baseline in ADP score demonstrated clinically meaningful (P<0.05) improvement with ZILRETTA compared with TAcs each week from Weeks 5 to 19 and compared with saline control each week from Weeks 1 to 20
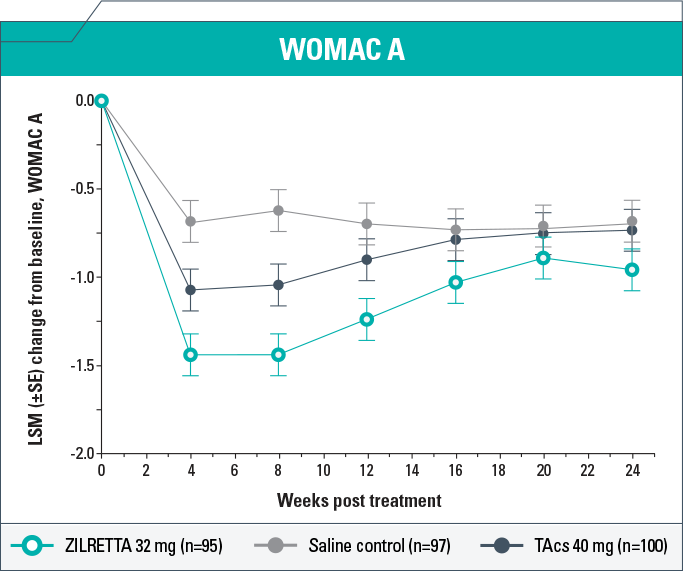
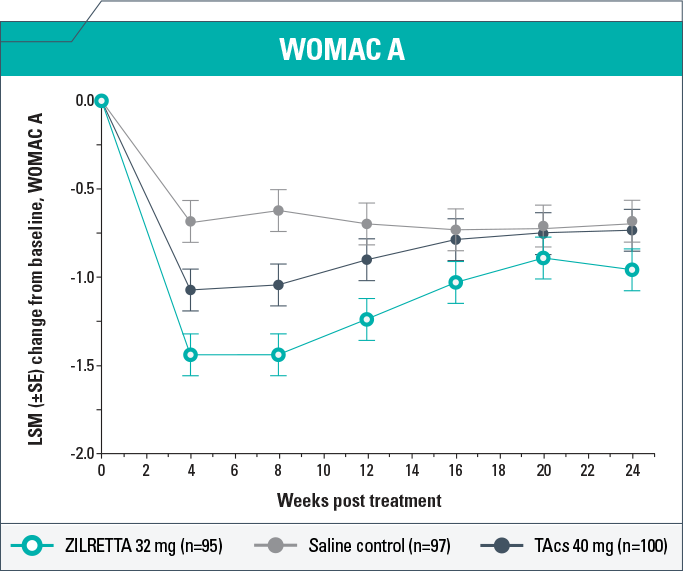
- ZILRETTA demonstrated clinically meaningful (P<0.05) improvements in WOMAC A scores compared with TAcs at Weeks 4, 8, and 12 and compared with saline control at Weeks 4, 8, 12, and 16
- ZILRETTA reduced WOMAC A pain scores from baseline by as much as 59% (Week 4); the largest reduction in pain resulting from TAcs treatment was 46% (Week 4)
Results from a post hoc analysis of a ZILRETTA Phase 3 trial
*Moderate to severe pain defined as ADP scores ≥5 to ≤9 (per
original inclusion criteria) and WOMAC A
scores ≥2.
ADP=average daily pain; TAcs=triamcinolone acetonide crystalline suspension; WOMAC=Western Ontario and McMaster Universities Osteoarthritis Index.
Among these consistent pain reporters, 28% of ZILRETTA patients reported no pain at Week 12, compared with 8% of TAcs patients3
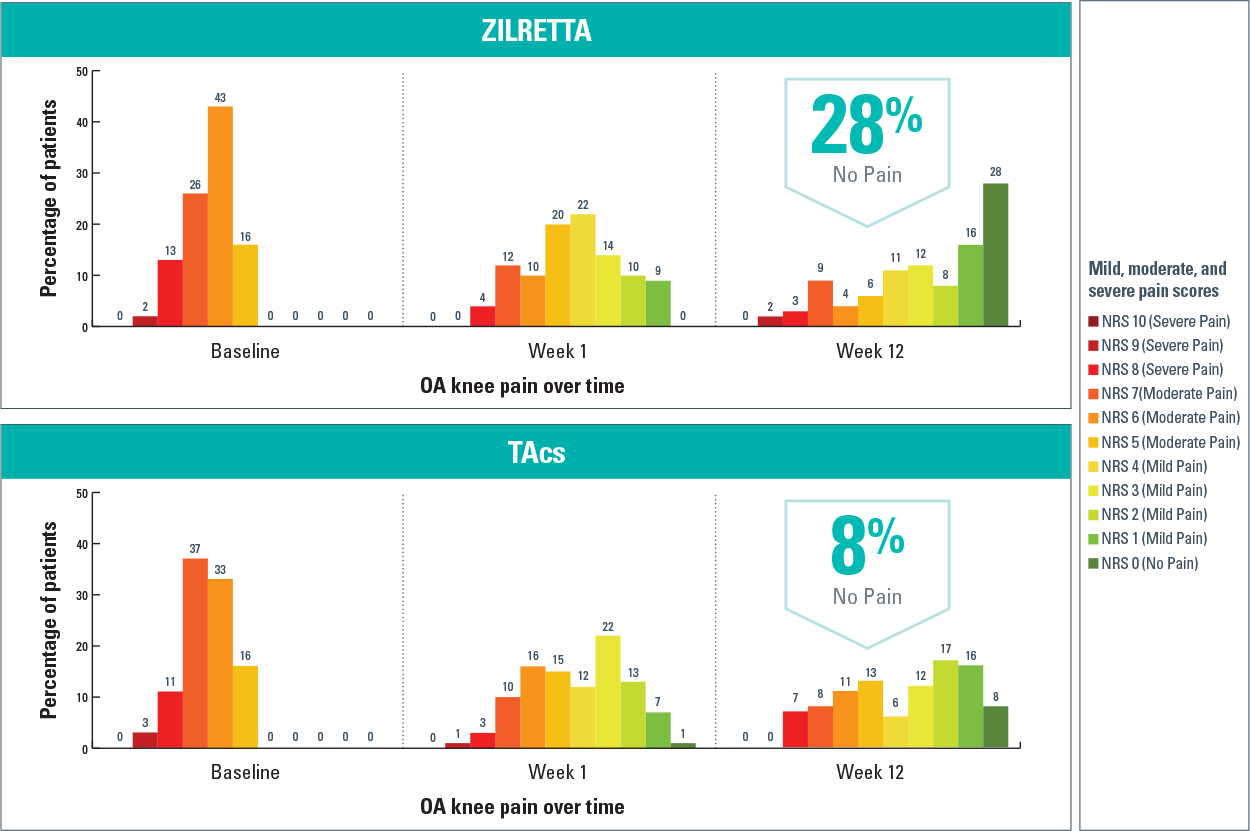
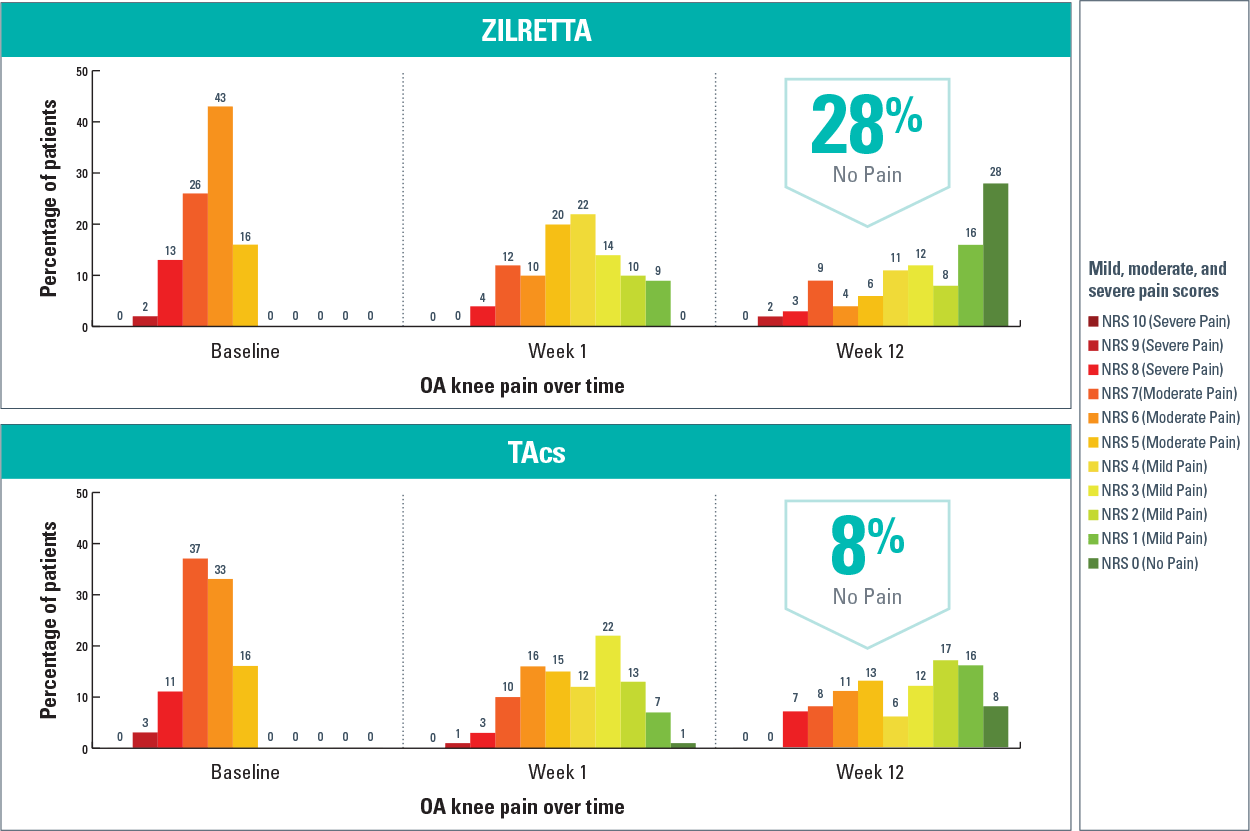
Results from a post hoc analysis of a ZILRETTA Phase 3 trial
ADP-intensity scores rated on a 0-10 NRS, with 0 indicating “no pain” and 10 indicating “pain as bad as you can imagine.”
ADP=average daily pain; NRS=numeric rating scale; TAcs=triamcinolone acetonide crystalline suspension.
